Case Study
Assisting our Client
with a Fast-Turnaround
Regulatory Request
from the FDA
The Question
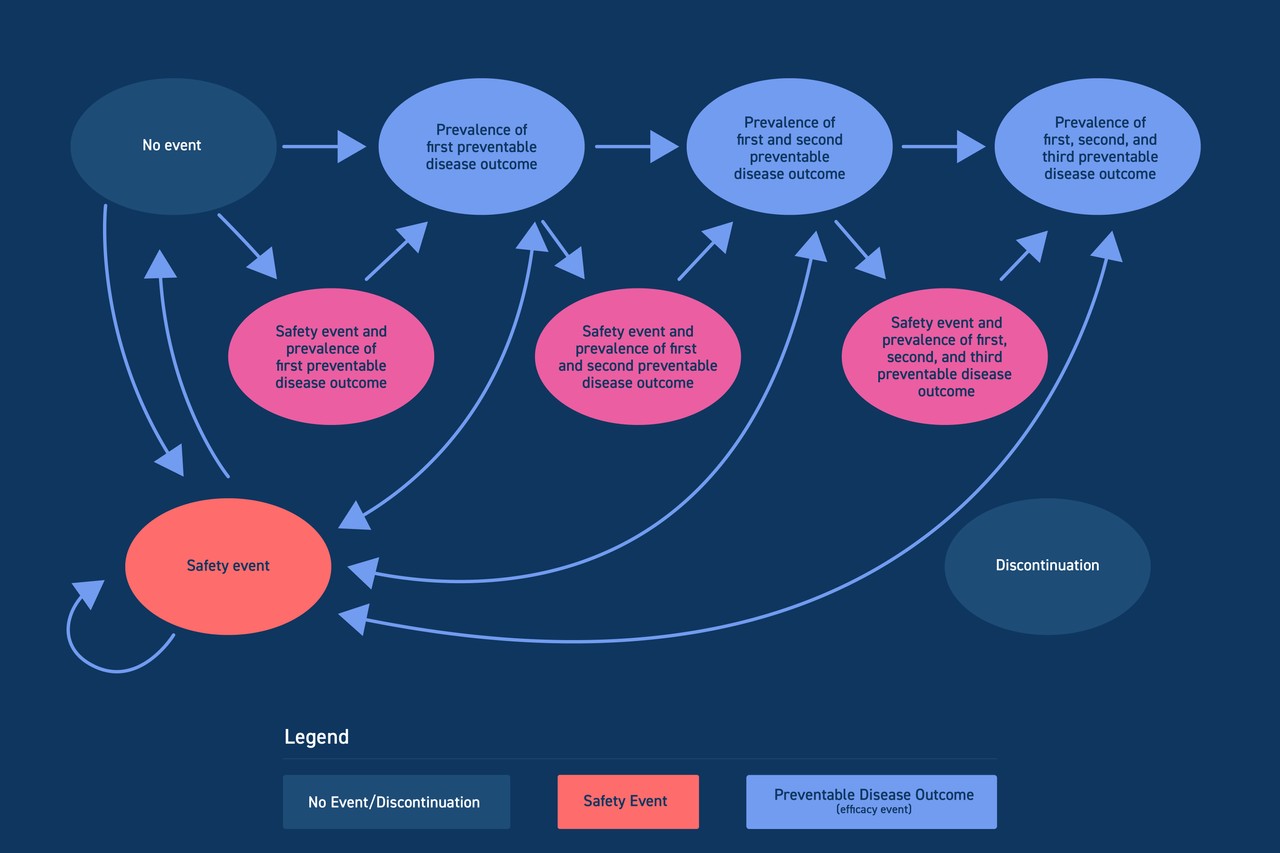
During regulatory review of their product, our client received a request from the FDA for a quantitative benefit-risk assessment that would provide additional analysis of their trial data for the purposes of understanding benefit / risk tradeoffs. The FDA suggested a Markov model be used.
The Approach
The Medicus team worked closely with the client’s HEOR and biostats teams, as well as an external KOL, to develop a Markov model that could tabulate safety and efficacy events and project them beyond the trial time horizon. Medicus analyzed the clinical trial data, developed the Markov model, and produced a report supporting the analysis and modeling decisions.
The Results
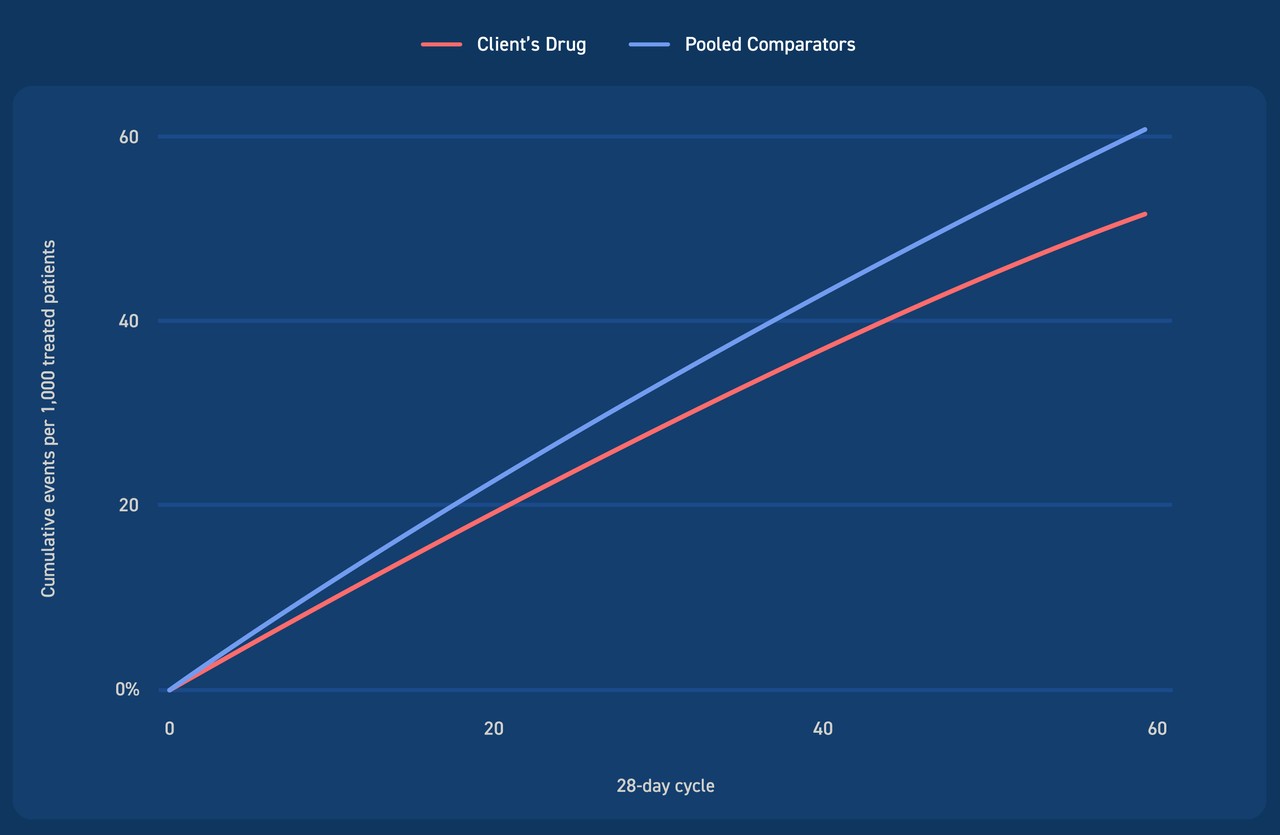
Under base assumptions, the model showed similar rates of cumulative preventable disease outcomes when projected over a five-year period. Sensitivity and scenario analyses showed results were robust to changes in model structure and input assumptions related to duration of treatment.
The Long And
Short Of it
Within one month after receiving the FDA request, the client was able to deliver a response to the FDA that included projections over a time horizon beyond the clinical trial, as well as transparent code and data as requested by the FDA.